An Introduction to pyGeochemCalc
Adedapo Awolayo and Benjamin Tutolo 2022-01-31
Overview
The pyGeochemCalc (pygcc) tool, a package for the python software environment, serves as an upgrade over the DBCreate and SUPCRT92 software package. DBCreate has been developed based on SUPCRT92 as a tool for creating thermodynamic database for geochemical calulations in tools like GWB, EQ36 and ToughReact (Kong et al., 2013). This community-driven, open-source Python package incorporates existing and newly implemented functions for calculating the thermodynamic properties of gas, aqueous, and mineral (including solid solutions - plagioclase, alkali-feldspar, olivine, pyroxene and clinopyroxene, and variable-formula clays) species, as well as reactions amongst these species, over a broad range of temperature and pressure conditions using the International Association for the Properties of Water and Steam (IAWPS) Formulation 1995 (Wagner and Pruß, 2002) equation of state and Zhang & Duan model Formulation (Zhang and Duan, 2005 and 2009) for Deep Earth Water (DEW) conditions. In addition, pygcc incorporates functions that permit the seamless integration and usage of these newly produced thermodynamic data in popular geochemical software including the Geochemist’s Workbench, EQ3/6, TOUGHREACT, and PFLOTRAN. The package is swift for ordinary stand-alone calculations and could be modified to support direct incorporation into other software, such as reactive-transport codes. The package is open-source and intended to be community-driven, such that the package and its capabilities can evolve in tandem with the needs of the geochemical modeling community
Installing and loading pyGeochemCalc
After starting python, install pygcc through running pip install pygcc in either your shell or anaconda prompt. Then, do either of the following as applicable:
create a python file (either .py or ipynb) and write the following command:
from pygcc.pygcc_utils import *
Save and Run the script with Spyder/Jupyter or in your shell/powershell/Anaconda prompt (if script is .py) using:
python scriptname.py
run python in your shell/powershell/Anaconda prompt by calling
pythonand then run the following command:
from pygcc.pygcc_utils import *
Getting help
After installation, type and run the following commands on Spyder terminal or Jupyter or shell/powershell/Anaconda prompt to browse the main function names in the pyGeochemCalc:
import pygcc.pygcc_utils as pyGCC
print(main_function_name(pyGCC))
['Helgeson_activity', 'Henry_duan_sun', 'OrderedDict', 'ZhangDuan', 'aw_correlation', 'calcRxnlogK', 'calc_elem_count_molewt', 'calclogKclays', 'co2fugacity', 'convert_temperature', 'db_reader', 'derivative', 'drummondgamma', 'gamma_correlation', 'heatcap', 'iapws95', 'importconfile', 'info', 'outputfmt', 'readIAPWS95data', 'roundup_hundredth', 'roundup_tenth', 'solidsolution_thermo', 'supcrtaq', 'water_dielec', 'write_database']
Type help(function name) on the commands to see an overview documentation for each function; many of which include examples. For example:
help(Helgeson_activity)
Help on function Helgeson_activity in module pygcc.pygcc_utils:
Helgeson_activity(TC, P, I, Dielec_method=None, **rhoEDB)
This function calculates the solute activity coefficient, solvent osmotic coefficient,
and solvent activity at given temperature and pressure using equations 298, 190 and 106 in
Helgeson, Kirkham and Flowers, 1981, A.J.S. p.1249-1516
Parameters:
----------
TC : Temperature [°C]
P : pressure [bar]
I : ionic strength
Dielec_method : specify either 'FGL97' or 'JN91' or 'DEW' as the method to calculate dielectric
constant (optional), if not specified default - 'JN91'
rhoEDB : dictionary of water properties like density (rho), dielectric factor (E) and
Debye–Hückel coefficients (optional)
Returns:
----------
aw : solvent activity
phi : solvent osmotic coefficient
mean_act : solute activity coefficient
Usage
-------
[aw, phi, mean_act] = Helgeson_activity( TC, P, I)
Description and Organization of major accompanied datasets
pyGeochemCalc contains the following major datasets and folders:
clay_elements.dat contain 42 clay names and fractional elemental composition of the clay mineral arranged in this specific order [‘Si’, ‘Al’, ‘FeIII’, ‘FeII’, ‘Mg’, ‘K’, ‘Na’, ‘Ca’, ‘Li’]. The function
calclogKclaysorcalcRxnlogKuses this dataset to estimate the thermodynamic properties of the clay minerals to be included in the newly-generated database.default_db is a folder that contains default thermodynamic databases utilized in several calculations in pygcc in case the user failed to provide any database. It can be categorized into:
direct-access:
speq21.dat is the sequential-access database that contains 1604 species thermodynamic properties, which undergoes timely update as newer information becomes available.
berman.dat is the sequential-access database that contains Berman mineral species thermodynamic properties.
supcrtbl.dat is the sequential-access database that contains Holland and Power (1998, 2011) mineral species thermodynamic properties gathered by Zimmer et al. (2016)
source:
data0.dat is a sample source thermodynamic database for EQ3/6
thermo.com.dat and thermo.com.tdat and thermo_latest.tdat are sample source thermodynamic databases for older (oct94), previous (apr20) and latest (mar21) formats of GWB respectively. Only these legacy dataset formats “apr20”, “jan19”, “jul17”, and “oct94” are currently supported by pygcc.
database is a folder that contains several other databases including files in default_db that could be used with pygcc
data0.geo, thermo.29Sep15.dat, and slop07.dat and dpumn2012.dat are example databases (first is an EQ3/6 source database, second is a GWB source database and last two are sequential-access and direct-access) used for testing purposes.
data0.hmw, thermo_hmw.tdat, and thermo_ymp.R2.tdat are example Ptifer databases (first is the EQ3/6 source database, and last two are GWB source databases) used for testing purposes.
Thermodynamic database
Properties of Database and reactions
The direct-access or sequential-access thermodynamic database can be read into a dictionary through the following commands:
Db_thermo = db_reader(dbaccess = './database/speq21.dat')
The function db_reader(dbaccess = None, dbBerman_dir = None, dbHP_dir = None) requires the users to specify the file location, name and its extension, of the direct-access or sequential-access thermodynamic database, if nothing is specified, the function reads the default database, speq21.dat. The dbBerman_dir input only needs to specified if the user(s) prefers Berman dataset for mineral species, then the filename and location needs to be specified (see dbBerman_dir = ‘./database/berman.dat’). Similarly, the dbHP_dir input (see dbHP_dir = ‘./database/supcrtbl.dat’) should only be included if the user(s) prefers Holland and Powell dataset for mineral and gas species. The above function returns two parameters; a dictionary containing the thermodynamic properties of each species (gas, minerals, aqueous and redox) within the direct-access/sequential-access thermodynamic database and a string containing the filename without the file location. The format of this dictionary contains a list, for example for a mineral using Maier-Kelley power function with no phase transition, as follows:
Db_thermo.dbaccessdic[‘mineral name’] = [‘formula’, ‘source date’, dG[cal/ml], dH[cal/mol], S[cal/mol-K], V[cm$^{3}$/mol], a[cal/mol-K], b[*10$^{3}$ cal/mol/K$^{2}$], c[*10$^{-5}$ cal/mol/K], Ttrans [K]]
For example, we can output the thermo properties of 'Diopside' with:
print(Db_thermo.dbaccessdic['Diopside'])
print(Db_thermo.dbaccess)
['CaMg(SiO3)2', ' ref:1,19 15.Mar.90\n', -723780.0, -765378.0, 34.2, 66.09, 52.87, 7.84, -15.74, 1600.0]
speq21.dat
Note:
‘speq21.dat’ contains properties in the following format - for minerals species (dG [cal/mol], dH [cal/mol], S [cal/mol-K], V [cm$^{3}$/mol], a [cal/mol-K], b [*10$^{3}$ cal/mol/K$^{2}$], c [*10$^{-5}$ cal/mol/K], Ttrans [K], Htr [cal/mol], Vtr [cm$^{3}$/mol], dPdTtr [bar/K] ) and aqueous species (dG [cal/mol], dH [cal/mol], S [cal/mol-K], V [cm$^{3}$/mol], a1 [*10 cal/mol/bar], a2 [*10$^{-2}$ cal/mol], a3 [cal-K/mol/bar], a4 [*10$^{-4}$ cal-K/mol], c1 [cal/mol/K], c2 [*10$^{-4}$ cal-K/mol], ω [*10$^{-5}$ cal/mol] );
‘Berman.dat’ contains properties in the following format - for mineral species (dG [J/mol], dH [J/mol], S [J/mol-K], V [cm$^{3}$/mol], k0, k1, k2, k3, v1 [*10$^{5}$ K$^{-1}$], v2 [*10$^{5}$ K$^{-2}$], v3 [*10$^{5}$ bar^-1], v4 [*10$^{8}$ bar^-2], dTdP [K/bar], Tlambda [K], Tref [K], l1 [(J/mol)$^{0.5}$/K], l2 [(J/mol)$^{0.5}$/K$^{2}$], DtH, d0 [J/mol], d1 [J/mol], d2 [J/mol], d3 [J/mol], d4 [J/mol], d5 [J/mol], Tmin [K], Tmax [K])
‘supcrtbl.dat’ contains properties in the following format - for mineral species (dG [kJ/mol], dH [kJ/mol], S [J/mol-K], V [J/bar], a [kJ/mol-K], b [*10^5 kJ/mol/K^2], c [kJ-mol-K], d [kJ/mol/K^0.5], alpha [*10^5 K^-1], kappa0 [kbar], kappa0_d [kbar], kappa0_dd [kbar], n_atom [-], Tc0 [K], Smax [J/mol-K], Vmax [J/bar], dH [KJ/mol], dV [J/bar], W [kJ/mol], Wv [J/bar], n [-], SF [-])
If the user wishes to utilise the default database but needs to add any species properties that aren’t already there, a quick way is to follow the above commands and add the properties of the new species. For example:
Db_thermo.dbaccessdic[‘Diopside_new’] = [‘CaMg(SiO3)2’, ‘ ref:n/a 15.Mar.21’, -724780.0, -765878.0, 34.3, 66.19, 52.87, 7.84, -15.74, 1600.0]
Similarly, the source thermodynamic database for either EQ3/6 or GWB can be read and used in various calculations using the commands below:
# for GWB database
Db_thermo = db_reader(sourcedb = './database/thermo.com.dat', sourceformat = 'GWB'
#, dbBerman_dir = './database/berman.dat'
#, dbHP_dir = './database/supcrtbl.dat'
)
Another option is to refer to the name of a default source database, such as 'thermo.com', 'thermo.2021', and 'thermo_latest', as shown below
Db_thermo = db_reader(sourcedb = 'thermo.com', sourceformat = 'GWB')
The function db_reader(dbaccess = None, dbaccess_codecs = None, dbBerman_dir = None, dbHP_dir = None, sourcedb = None, sourceformat = None, sourcedb_codecs = None)above returns six parameters; a dictionary containing the reaction coefficient and species from the source thermodynamic database, a list of list categorizing the species in the source database into basis gas, minerals, aqueous and redox species, a dictionary containing the charges of each species, a dictionary containing the molecular weight of each species, a dictionary containing the mineral type of each mineral species, a list containing fugacity info for gas species, required for the latest GWB format and a dictionary containing the activity coefficient parameters, especially with Pitzer activity model. The codecs input for both or either of the databases (dbaccess_codecs or sourcedb_codecs) specify the name of the encoding used to decode or encode the respective files. Most files are encoded as utf-8 as it is an ASCII-compatible encoding that can encode any valid Unicode code point, hence, it is made the default encoding name. See the documentation for more information. The format of the dictionary containing reaction coefficient and species for GWB is as follows:
print(Db_thermo.sourcedic['Diopside'])
[' CaMgSi2O6', 5, '-4.0000', 'H+', '1.0000', 'Ca++', '1.0000', 'Mg++', '2.0000', 'H2O', '2.0000', 'SiO2(aq)']
Search function
Suppose the user is interested in finding the naming system for a particular species, this function searches through any of the database (either direct-access or source) for any name that contains the declared string and returns species that matching the search. This is highly critical when it is important to know exactly how the species name is written within each of the database to get the right match for various geochemical calculations, such as thermodynamic properties or chemical reaction. For example, gather all species containing HCO$_{3}$ in its compositions from source dictionary:
print(info('HCO3', Db_thermo.sourcedic))
['HCO3-', 'HCO3-', '(UO2)3O(OH)2(HCO3)+', 'CaHCO3+', 'CdHCO3+', 'CeHCO3++', 'DyHCO3++', 'ErHCO3++', 'EuHCO3++', 'EuOHCO3(aq)', 'FeHCO3+', 'GdHCO3++', 'HoHCO3++', 'LaHCO3++', 'LuHCO3++', 'MgHCO3+', 'MnHCO3+', 'NaHCO3(aq)', 'NdHCO3++', 'PmHCO3++', 'PrHCO3++', 'SmHCO3++', 'TbHCO3++', 'TmHCO3++', 'YHCO3++', 'YbHCO3++', 'ZnHCO3+', 'AmOHCO3', 'EuOHCO3', 'NdOHCO3']
This function can also be used to identify the available species that contain particular chemical elements. For example, to get Rubidium-bearing species for direct-access database, run this:
print(info('Rb', Db_thermo.dbaccessdic))
['Rb+', 'RbBr(aq)', 'RbCl(aq)', 'RbF(aq)', 'RbI(aq)', 'RbOH(aq)', 'Rb(Ac)(aq)', 'Rb(Ac)2-', 'Rb+', 'RbBr(aq)', 'RbCl(aq)', 'RbF(aq)', 'RbI(aq)', 'RbOH(aq)', 'Rb(Ac)(aq)', 'Rb(Ac)2-']
Water Equation of State
IAPWS95 (Wagner and Pruß, 2002) and FGL (Fernández et al., 1997) formulations are implemented in pygcc for the calculation of the thermodynamic and dielectric properties of water and steam, while the ZhangDuan (Zhang and Duan, 2005 and 2009) and DEW (Sverjensky et al., 2014) formulations are implemented for high temperature and pressure conditions. However since many of the coefficients in the HKF equations (especially dielectric constant) required to calculate thermodynamic properties of other species are based on the IAPWS84 formulation (popularly known as HGK84 or NBS/NRC-84, Haar et al. (1984)) incorporated in the H2O92 subroutine from SUPCRT92 (Johnson et al., 1992) and to avoid regressing those parameters, Johnson and Norton formulation for dielectric constant (Johnson and Norton, 1991) was implemented in pygcc. Hence, the users have the option of choosing either formulation (FGL97 or JN91) for dielectric constant calculation. Other water properties are calculated using the IAPWS95 formulation, in part because of the intrinsic thermodynamic differences between IAPWS84 and IAPWS95, and also that the IAPWS95 equations can be extrapolated to a greater range of Temperatures and Pressures than IAPWS84. In addition, .
IAPWS-95 Formulation
The function iapws95(T = None, P = None, rho = None, rho0 = None, FullEOSppt = False, v = None, Out_Unit = 'standard') is designed to take a combination of different inputs - temperature [°C], density [kg/m$^{3}$], pressure [bar] and output equation of state properties such as pressure [bar], Helmholtz energy [cal/mol/K or kJ/kg/K], Entropy [cal/mol/K or kJ/kg/K], Enthalpy [cal/mol or kJ/kg], Gibbs energy [cal/mol or kJ/kg], Volume [m$^{3}$/mol or m$^{3}$/kg], Derivative of pressure with respect to delta [bar], Helmholtz energy derivative with respect to reduced density (delta), zeta value (needed to calculate viscosity), Derivative of pressure with respect to reduced temperature (tau) [bar], Compressibility [/bar], Thermal expansion coefficient (thermal expansivity). Other water properties such as viscosity [Pa-s], Internal energy [cal/mol or kJ/kg], Isobaric heat capacity [cal/mol/K or kJ/kg/K], Isochoric heat capacity [cal/mol/K or kJ/kg/K], Joule-Thomsen coefficient [K-m$^{3}$/kJ], Isothermal throttling coefficient [kJ/kg/bar], Isentropic temperature-pressure coefficient [K-m$^{3}$/kJ], Speed of sound [m/s] are returned as outputs only when FullEOSppt input is set as True as they are not currently utilized in any calculation in pygcc. Also, the Out_Unit has the option of converting the properties to 'standard' (calorie units) or ‘kilogram’ (kilogram units). Check the documentation for more information. There are three basic types of problems in evaluating this equation-of-state model and these problems are distinguished by their specified inputs as below:
Properties as a Function of Temperature and Density
This is a straightforward approach that does not require any iteration. Here, the temperature [°C] and density [kg/m$^{3}$] are specified as inputs in iapws95 and returns various thermodynamic properties as previously mentioned. An example of utilizing iapws95 for calculating properties at 500 K and 838.025 kg/m$^{3}$ is as follows:
water = iapws95(T = 226.85, rho = 838.025, Out_Unit='kilogram')
water.P, water.F, water.S, water.H, water.G, water.V
(array(100.00385801),
array(-318.20624717),
array(2.56690919),
array(977.18162414),
array(-306.27296857),
array(0.00119328))
water = iapws95(T = 226.85, rho = 838.025, Out_Unit='standard', FullEOSppt = True)
water.P, water.F, water.S, water.H, water.G, water.V, water.mu, water.Cp
(array(100.00385801),
-60206.765249165524,
26.189860494061474,
-64559.264477311226,
-61032.47210455878,
0.0051379794852494795,
array(0.00011983),
19.8160522598234)
Properties as a Function of Temperature and Pressure
This approach involves an iterative solution to the above approach by finding and revising the value of density that is consistent with the desired input pressure using Newton-Raphson method. The implementation of this approach is carried out by function iapws95 where a user-specified starting estimate of density (rho0) can be optionally utilized. The approached used in generating starting guess is similar to that described in Wolery (2020). The function iapws95 takes temperature [°C] and pressure [bar] as inputs and returns various thermodynamic properties. An example of utilizing iapws95 for calculating properties at 25 °C and 1 bar is as follows:
water = iapws95(T = 25, P = 1)
water.rho, water.S, water.H, water.G, water.V, water.P, water.TC
(array([997.04703902]),
array([16.71845103]),
array([-68315.01431914]),
array([-56677.89787508]),
array([0.00431851]),
array([1]),
array([25]))
Calculating Properties on the Saturation Curve
This approach deals with the calculation of thermodynamic properties on the saturation (vapor-liquid equilibrium) curve, extending from the triple point (273.16K, 0.00611657 bar) up to the critical point (647.096K, 220.64 bar) as show below.
import matplotlib.pyplot as plt
pp = np.linspace(0.00611657, 220.64, 50)
tt = np.linspace(0.01, 373.946, 50)
water = iapws95(P = pp)
fig = plt.figure()
ax = plt.axes(projection='3d')
ax.plot3D(tt - 273.15, pp, water.rhosl)
ax.plot3D(tt - 273.15, pp, water.rhosv, color='red')
ax.set_xlabel('Temperature [°C]')
ax.set_ylabel('Pressure [bar]')
ax.set_zlabel('Density [kg/m3]')
ax.tick_params(direction = 'in', which='both', top=True, right=True)
ax.grid(False)
ax.get_tightbbox(fig.canvas.get_renderer(), call_axes_locator = True, bbox_extra_artists = None)
/home/docs/checkouts/readthedocs.org/user_builds/pygcc/envs/latest/lib/python3.7/site-packages/scipy/optimize/minpack.py:175: RuntimeWarning: The iteration is not making good progress, as measured by the
improvement from the last ten iterations.
warnings.warn(msg, RuntimeWarning)
Bbox([[143.2, 52.8], [1392.0385135135134, 1241.5135135135133]])
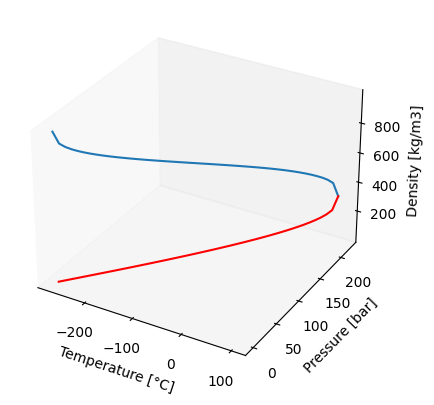
Here, the users can specify either a temperature or pressure value and the implementation of this type can solve for corresponding pressure (T) or temperature (P) respectively and their respective vapor and liquid densities. These functions have been implemented in iapws95 to calculate thermodynamic properties when saturation curve properties are requested. An example of utilizing iapws95 for calculating properties at temperatures ranging from 0.01°C to 300°C and saturation pressures is as follows:
TC = np.linspace(0.01, 200, 5)
water = iapws95(T = TC, P = 'T')
water.rho, water.G, water.H, water.S, water.V, water.P, water.TC
(array([999.84376462, 988.031656 , 958.34546327, 917.00540392,
864.65810229]),
array([-56279.53650436, -57114.34721375, -58089.12256378, -59184.20258644,
-60385.54751607]),
array([-68766.32498231, -67864.92957317, -66961.8516668 , -66044.71299022,
-65097.09778613]),
array([15.13740717, 18.1680458 , 20.76615218, 23.06783539, 25.1720857 ]),
array([0.00430643, 0.00435791, 0.00449291, 0.00469545, 0.00497972]),
array([ 1.0133 , 1.0133 , 1.01436095, 4.76196448, 15.549279 ]),
array([1.000000e-02, 5.000750e+01, 1.000050e+02, 1.500025e+02,
2.000000e+02]))
Meanwhile, for calculating water properties with specified pressure at saturation temperature, a typical usage is as follows:
water = iapws95(P = 200, T = 'P')
water.rho, water.G, water.H, water.S, water.V, water.P, water.TC
(array([490.18783526]),
array([-64995.46289957]),
array([-60899.23505785]),
array([32.4275702]),
array([0.00878389]),
array([200]),
array([365.74925556]))
ZhangDuan Formulation
Zhang and Duan (2005) proposed an empirical pressure-volume-temperature equation of state for water at high temperature and pressure, which Sverjensky et al. (2014) developed into Deep Earth Water model. Its implementation in pygcc takes the first two forms described above, which are estimating Properties as a Function of Temperature and Pressure and Properties as a Function of Temperature and Density. However, in both cases, the outputs are density [kg/m$^{3}$]/pressure [bar], Gibbs energy [cal/mol], derivative of density to pressure at constant temperature and derivative of density to temperature at constant pressure. The typical usage of the ZhangDuan() function is as below:
water = ZhangDuan(T = 250, P = 1000)
water.rho, water.G, water.drhodP_T, water.drhodT_P
(array([875.09805237]),
array([-61195.9629225]),
array([9.3131885e-05]),
array([-0.00102445]))
Dielectric Constants Calculation
The dielectric constant is one of the most critical properties of water required in calculating Born functions, Debye-Hückel parameters and molal properties of species and reactions at varying temperature and pressure conditions. Like earlier noted, two different formulations were implemented for non-extreme temperature and pressure conditions, and users have a choice to pick the preferred formulation using Dielec_method and as further described below. The function water_dielec() takes any two combinations of temperature [°C], pressure [bar] and density [kg/m$^{3}$] as inputs, and returns various properties of water such as dielectric constant of water [cal/mol], density [g/cm$^{3}$], Debye-Huckel “A” parameters [kg$^{1/2}$ mol$^{-1/2}$], Debye-Huckel “B” parameters [kg$^{1/2}$ mol$^{-1/2}$ Angstrom$^{-1}$], bdot, Debye-Huckel “A” parameters derivatives associated with apparent molar enthalpy and apparent molar volume, and Debye-Huckel “B” parameters derivatives associated with apparent molar enthalpy and apparent molar volume.
FGL Electrostatic Formulation
The IAPWS-sanctioned model for the dielectric constant of water is described in IAPWS (1997) and the relevant equations and constants for the model are given in the source document and the paper by Fernández et al. (1997). An example of utilizing FGL97 for calculating properties at 50°C and 1.0133 bar (at saturation curve) is as follows:
dielect = water_dielec(T = 50, P = 'T', Dielec_method = 'FGL97')
dielect.E, dielect.rhohat, dielect.Ah, dielect.Bh, dielect.bdot, dielect.Adhh, dielect.Adhv, dielect.Bdhh, dielect.Bdhv
(array([69.91599636]),
array([0.98803505]),
array([0.53410832]),
array([0.3325671]),
array([0.04088528]),
array([0.68757801]),
array([2.37680041]),
array([16.55676064]),
array([-13.40777756]))
JN91 Electrostatic Formulation
The dielectric constant equation as expressed by Johnson and Norton (1991) and utilized in SUPCRT92 is implemented here. An example of utilizing JN91 for calculating properties at 50°C and 1.0133 bar (at saturation curve) is as follows:
dielect = water_dielec(T = 50, P = 'T', Dielec_method = 'JN91')
dielect.E, dielect.rhohat, dielect.Ah, dielect.Bh, dielect.bdot, dielect.Adhh, dielect.Adhv, dielect.Bdhh, dielect.Bdhv
(array([69.80673495]),
array([0.98803505]),
array([0.53537265]),
array([0.33283636]),
array([0.04088528]),
array([0.68352145]),
array([2.53510753]),
array([16.39347606]),
array([-18.16464841]))
DEW Electrostatic Formulation
Sverjensky et al (2014) extended the application of the revised HKF model to deep Earth (upper mantle) conditions by developing an empirical correlation for dielectric constant of water up to 60 kbar and 1200 °C using experimental data and molecular dynamics simulation data. This implementation is considered with ZhangDuan water EoS to model high temperature and pressure conditions, taking inputs and printing output similar to the above description. An example of the utilization of DEW to calculate properties at 200 °C and 1000 bar is as follows:
dielect = water_dielec(T = 200, P = 1000, Dielec_method = 'DEW')
dielect.E, dielect.rhohat, dielect.Ah, dielect.Bh, dielect.bdot, dielect.Adhh, dielect.Adhv, dielect.Bdhh, dielect.Bdhv
(array([38.32009939]),
array([0.92183148]),
array([0.71764521]),
array([0.35859842]),
array([0.04452579]),
array([0.2115715]),
array([7.02321141]),
array([52.29367986]),
array([-66.08043662]))
Calculating thermodynamic properties
The molal properties of species and reactions at any temperature and pressure condition are computed using the different classification described below.
Thermodynamic Properties of Reactions among species
The calculation of equilibrium constants for reactions among aqueous, gas, and mineral species is implemented in the pygcc calcRxnlogK(T = None, P = None, Specie = None, dbaccessdic = None, sourcedic = None, specielist = None, heatcap_method = None, sourceformat = None, densityextrap = False) function. The logarithm (base 10) of the equilibrium constant ($ K_{eq} $) of any reaction is calculated via:
$$log K_{eq} = \frac{G^{reactants}{T, P} - G^{products}{(T, P)}}{-RTln(10)} $$
The function outputs logarithm of the equilibrium constant, Gibbs free energy of the reaction, total Gibbs free energy of the product species and total Gibbs free energy of the reactant species. The required inputs are temperature [°C], pressure [bar], Specie (a string that specifies the name of the product species), direct-access database dictionary (for example above, Db_thermo.dbaccessdic), source database dictionary and species list (for example above, Db_thermo.sourcedic, Db_thermo.specielist), densityextrap (bool - True or False - to indicate usage of density extrapolation), sourceformat (either GWB or EQ36) and heatcap_method (specifying either 'SUPCRT' or 'HF76' or 'HP11' or 'Berman88' for using either Maier-Kelley powerlaw, Haas-Fisher powerlaw, Holland and Powell and Berman’s formulation function respectively) and other optional inputs like Dielec_method, ThermoInUnit, Al_Si, Specie_class, ClayMintype, elem, group, cpx_Ca, X and rhoEGextrap. Check the documentation for more information.
Redox and Aqueous species
The standard molal Gibbs energy of aqueous, redox or auxilliary aqueous species with temperature and pressure can be calculated using the supcrtaq function. The name and the different equations implemented in this function is derived from the SUPCRT package (Johnson et al., 1992). An example of utilizing supcrtaq for calculating Gibbs free energy of ‘NaCl(aq)’ at 60°C and 100 bar with different Dielec_method and higher pressure DEW is as follows:
supcrtaq(60, 100, Db_thermo.dbaccessdic['NaCl(aq)'], Dielec_method = 'JN91')
array([-93847.85528561])
supcrtaq(60, 100, Db_thermo.dbaccessdic['NaCl(aq)'], Dielec_method = 'FGL97')
array([-93847.82866489])
supcrtaq(100, 1000, Db_thermo.dbaccessdic['NaCl(aq)'], Dielec_method = 'DEW')
array([-94480.46872573])
Mineral and gas species
The standard molal Gibbs free energy and heat capacity with temperature and pressure of mineral and gas species can be calculated in pygcc using the heatcap function based on Maier-Kelly powerlaw (Maier and Kelley, 1932), Haas-Fisher powerlaw (Haas and Fisher 1976), and formulation by Holland and Powell (1998) and Berman (1988). The 5-term Haas-Fisher polynomial equation (Haas and Fisher 1976) was implemented to calculate Gibbs free energy and heat capacity for some minerals as reported by Robie and Hemingway (1995), as implemented by setting the method to HF78. In addition, Berman (1988) reported an internally consistent standard state thermodynamic data set for 67 minerals using a different thermodynamic conventions as compared with that used by Helgeson et al. (1978). The equation of state by Berman (1988) was converted to the Helgeson Convention used in pygcc by considering the equation below as implemented in heatcap function by setting the method to berman88.
$$ G_{Helgeson} = G_{Berman} + T_{r} S^{elements}_{(Tr, Pr)} $$
Because it has been established that expansibilities and compressibilities can have considerable influence on computed Gibbs free energies of minerals, Holland and Powell (1998) recently proposed an equation of state that handles mineral volumes as temperature and pressure dependent. This function can be utilized by setting the method to HP11 as implemented in the heatcap function. Examples of utilizing heatcap for calculating Gibbs free energy and heat capacity of ‘CO$_{2}$(g)’, ‘Fluorapatite’, and ‘Quartz’ respectively at 60°C and 100 bar with the default JN91 Dielec_method are as follows:
sp = heatcap(T = 60, P = 100, Species = 'CO2(g)', Species_ppt = Db_thermo.dbaccessdic['CO2(g)'], method = 'SUPCRT')
sp.dG, sp.dCp
(array([-96059.91196724]), array([9.41357392]))
sp = heatcap(T = 60, P = 100, Species = 'Fluorapatite', Species_ppt = Db_thermo.dbaccessdic['Fluorapatite'], method = 'HF76')
sp.dG, sp.dCp
(array([-1554128.63647955]), array([94.71751487]))
ps = db_reader(dbBerman_dir = './database/berman.dat')
sp = heatcap( T = 60, P = 100, Species = 'Quartz', Species_ppt = ps.dbaccessdic['Quartz'], method = 'berman88')
sp.dG, sp.dCp
Duplicate found for species "Cordierite" in berman.dat
(array([-204972.30130472]), array([11.51694157]))
db = db_reader(dbHP_dir = './database/supcrtbl.dat')
sp = heatcap( T = 60, P = 100, Species = 'Quartz', Species_ppt = db.dbaccessdic['Quartz'], method = 'HP11')
sp.dG, sp.dCp
Duplicate found for species "Fluorphlogopite" in supcrtbl.dat
Duplicate found for species "Arsenic" in supcrtbl.dat
Duplicate found for species "Arsenolite" in supcrtbl.dat
(array([-204968.01944417]), array([11.53479289]))
Solid-solution species
The standard molal properties of solid-solution species and reactions are computed differently from other minerals, interaction parameters and mixing rules as described in Stefánsson (2001) are applied in combining the properties of various end-members thermodynamic data from Robie and Hemingway (1995). The function solidsolution_thermo or calcRxnlogK computes reaction species and coefficient, and molal properties of solid-solutions of plagioclase, olivine, pyroxene, alkali-feldspar and clinopyroxene minerals. The inputs are mole fractions of Anorthite, Forsterite, Enstatite or Albite as the case may be and number of moles of Calcium in the clinopyroxene formula units and the $\frac{Mg}{Mg+Fe}$ ratio for clinopyroxene, coupled with temperature [°C], pressure [bar], solidsolution_type (specifying either ‘All’ or ‘plagioclase’ or ‘olivine’ or ‘pyroxene’ or ‘cpx’ or ‘alk-feldspar’) and a direct-access dictionary (for example above, [Db_thermo.dbaccessdic]). If the moles of calcium is zero, the solid-solution is reduced to pyroxene end-member, which is is a binary solid solution between orthoenstatite and ferrosilite. Hence, setting the solidsolution_type to 'cpx' should only be used if moles of calcium is greater than zero. The structure of the output includes an array of equilibrium constants and a dictionary containing mineral type, name, formula, molecular weight, list of properties (like Db_thermo.dbaccessdic), reaction species and coefficient, mineral volume, data source and elements species and moles. An example that calculates solid-solution properties for ‘Di45Hed25En19Fe11’ at 60°C and 100 bar with the default JN91 formulation is:
ss = solidsolution_thermo(T = 60, P = 100, solidsolution_type = 'cpx', X = 0.7, cpx_Ca = 0.646,
dbaccessdic = Db_thermo.dbaccessdic)
ss.logK, ss.Rxn
(array([17.72530764]),
{'type': 'cpx',
'name': 'Di45Hed19En25Fe11',
'formula': 'Ca0.65Mg0.95Fe0.41Si2O6',
'MW': 224.20512,
'min': ['Ca0.65Mg0.95Fe0.41Si2O6',
' R&H95, Stef2001',
-681678.7564868213,
nan,
39.535095090743326,
65.566176,
95.86780066921605,
-0.01085667447418738,
-89154.23518164441,
-887.9544455066921,
3.040245219885277e-06],
'spec': ['H+', 'Ca++', 'Mg++', 'Fe++', 'SiO2(aq)', 'H2O'],
'coeff': [-4, 0.65, 0.95, 0.41, 2, 2],
'nSpec': 6,
'V': 65.566176,
'source': ' R&H95, Stef2001',
'elements': ['0.6500',
'Ca',
'0.9500',
'Mg',
'0.4100',
'Fe',
'2.0000',
'Si',
'6.0000',
'O']})
A similar result can be generated using [calcRxnlogK] as below and the difference is that calcRxnlogK allows computation of equilibrium constants using density extrapolation as below .
ss = calcRxnlogK(T = 60, P = 100, Specie = 'cpx', X = 0.7, cpx_Ca = 0.646, dbaccessdic = Db_thermo.dbaccessdic)
ss.logK, ss.Rxn
(array([17.72530764]),
{'type': 'cpx',
'name': 'Di45Hed19En25Fe11',
'formula': 'Ca0.65Mg0.95Fe0.41Si2O6',
'MW': 224.20512,
'min': ['Ca0.65Mg0.95Fe0.41Si2O6',
' R&H95, Stef2001',
-681678.7564868213,
nan,
39.535095090743326,
65.566176,
95.86780066921605,
-0.01085667447418738,
-89154.23518164441,
-887.9544455066921,
3.040245219885277e-06],
'spec': ['H+', 'Ca++', 'Mg++', 'Fe++', 'SiO2(aq)', 'H2O'],
'coeff': [-4, 0.65, 0.95, 0.41, 2, 2],
'nSpec': 6,
'V': 65.566176,
'source': ' R&H95, Stef2001',
'elements': ['0.6500',
'Ca',
'0.9500',
'Mg',
'0.4100',
'Fe',
'2.0000',
'Si',
'6.0000',
'O']})
Clay Mineral species
The thermodynamic properties for any clay minerals, including 42 of which are compiled in clay_elements.dat, can be calculated based on techniques detailed by Blanc et al (2015, 2021). These techniques have been implemented in function calclogKclays, which takes inputs as temperature [°C], pressure [bar], a list containing clay mineral name and elemental composition in this particular order [‘clay_name’, ‘Si’, ‘Al’, ‘FeIII’, ‘FeII’, ‘Mg’, ‘K’, ‘Na’, ‘Ca’, ‘Li’], a dictionary containing the thermodynamic properties of each species (for example above, [Db_thermo.dbaccessdic]) and a string that specifies the structural layering of the phyllosilicate. Examples of utilizing calclogKclays and calcRxnlogK for calculating properties of ‘Chamosite’ at 60°C and 100 bar with the default Dielec_method are:
calclogKclays(60, 100, *['Chamosite','3','2','0','5','0','0','0','0','0'],
dbaccessdic = Db_thermo.dbaccessdic, group='14A')
(array([39.88038017]),
{'type': 'Smectites',
'name': 'Chamosite',
'formula': 'Fe5.00Al2.00Si3.0O10(OH)8',
'MW': 713.5073000000001,
'min': ['Fe5.00Al2.00Si3.0O10(OH)8',
'B2015, B2021',
-1553442.9682398445,
-1699939.053537285,
134.53834562298331,
215.92,
146.64913957934988,
86.36591778202677,
-30.076481835564053],
'spec': ['H+', 'Fe++', 'Al+++', 'SiO2(aq)', 'H2O'],
'coeff': [-16.0, 5.0, 2.0, 3.0, 12.0],
'nSpec': 5,
'V': 215.92,
'dG': -6499.60537911551,
'dHf': -7112.545000000001,
'Cp': 579.765,
'source': 'B2015, B2021',
'elements': ['5.0000',
'Fe',
'2.0000',
'Al',
'3.0000',
'Si',
'8.0000',
'H',
'18.0000',
'O']})
clay = calcRxnlogK(T = 60, P = 100, elem = ['Chamosite','3','2','0','5','0','0','0','0','0'],
Specie = 'clay', dbaccessdic = Db_thermo.dbaccessdic)
clay.logK, clay.Rxn
(array([39.88038017]),
{'type': 'Smectite',
'name': 'Chamosite',
'formula': 'Fe5.00Al2.00Si3.0O10(OH)8',
'MW': 713.5073000000001,
'min': ['Fe5.00Al2.00Si3.0O10(OH)8',
'B2015, B2021',
-1553442.9682398445,
-1699939.053537285,
134.53834562298331,
215.92,
146.64913957934988,
86.36591778202677,
-30.076481835564053],
'spec': ['H+', 'Fe++', 'Al+++', 'SiO2(aq)', 'H2O'],
'coeff': [-16.0, 5.0, 2.0, 3.0, 12.0],
'nSpec': 5,
'V': 215.92,
'dG': -6499.60537911551,
'dHf': -7112.545000000001,
'Cp': 579.765,
'source': 'B2015, B2021',
'elements': ['5.0000',
'Fe',
'2.0000',
'Al',
'3.0000',
'Si',
'8.0000',
'H',
'18.0000',
'O']})
Density extrapolation
The calculation of the standard molal Gibbs free energy of charged species as described by the revised Helgeson-Kirkham-Flowers (HKF) equation of state is limited to temperature-pressure condition for which the water density > 0.35 g/$cm^{3}$, temperature < 350°C, pressure < 500 bar and not valid within the critical water region (350 – 400 °C). Hence, outside this region, the approach that is considered here is to linearly extrapolate thermodynamic properties with 0.35 g/$cm^{3}$ < density < 0.6 g/$cm^{3}$ to calculate corresponding equilibrium constants for region with density < 0.35 g/$cm^{3}$ as show below. However, regions where density > 0.35 g/$cm^{3}$ and critical water region (350 – 400 °C) are still not valid within this density extrapolation range.
from density_extrap import plot_densityextrap
plot_densityextrap()
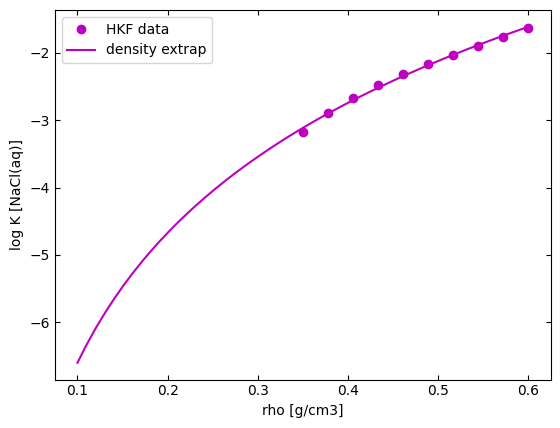
This approach is implemented in calcRxnlogK and can be activated by setting densityextrap to True. Examples are shown below
rxncalc = calcRxnlogK(T = 450, P = 300, Specie = 'H2S(aq)', sourcedic = Db_thermo.sourcedic,
dbaccessdic = Db_thermo.dbaccessdic, specielist = Db_thermo.specielist,
densityextrap = True)
rxncalc.logK
array([-14.26282955])
ss = calcRxnlogK(T = 450, P = 300, Specie = 'plagioclase', X = 0.7, densityextrap = True,
dbaccessdic = Db_thermo.dbaccessdic)
ss.logK
array([14.31769536])
clay = calcRxnlogK(T = 450, P = 300, elem = ['Chamosite','3','2','0','5','0','0','0','0','0'],
Specie = 'clay', dbaccessdic = Db_thermo.dbaccessdic, group = '14A',
densityextrap = True)
clay.logK
array([41.68113239])
Generating thermodynamic database
Generating database for any of GWB, EQ3/6, ToughReact, Pflotran or Phreeqc (under development) can be easily achieved through the function write_database. This function requires several inputs some of which are compulsory - temperature [°C], pressure [bar] and dataset format (GWB, EQ36, ToughReact, or Pflotran). Note: GWB requires exactly 8 temperature and pressure pairs. If the users want default solid_solution and clay_minerals included, then the inputs have to include ‘Yes’ or True option. By default, density extrapolation is included to generate equilibrium constants for regions outside the revised HKF applicability, however, if the users want to turn off density extrapolation, this can be achieved by setting densityextrap = 'No'. Meanwhile, for ToughReact and Pflotran, their source database can be either of GWB or EQ3/6 and this option needs to included as part of the inputs through ‘sourceformat’, see example below.
A typical example of the command to generate GWB using 0°C to 350°C temperature range, 300 bar and nCa of 0.5 and turning on the inclusion of solid_solution and clay minerals thermodynamics is as follows:
write_database(T = [0, 350], P = 300, nCa_cpx = 0.5, solid_solution = 'Yes', clay_thermo = 'Yes', dataset = 'GWB')
Success, your new GWB database is ready for download
<pygcc.pygcc_utils.write_database at 0x7f05e41468d0>
You can scroll to the folder “output” to access the newly generated database as well as a text file “spxNotFound” containing species that are not found in direct-access database.
Another typical example (converting EQ3/6 to GWB) of the command to generate GWB using EQ3/6 source database, 0°C to 250°C temperature range, 100 bar and turning on the inclusion of solid_solution and clay minerals thermodynamics is as follows:
write_database(T = [0, 250], P = 100, solid_solution = True, clay_thermo = True,
dataset = 'GWB', sourcedb = './database/data0.geo', sourceformat = 'EQ36')
Success, your new GWB database is ready for download
<pygcc.pygcc_utils.write_database at 0x7f05e12c3790>
A typical example of the command to generate EQ3/6 using specified temperature range, 250 bar and nCa of 0.5 and turning on the inclusion of solid_solution thermodynamics only is as follows:
T = np.array([ 0.010, 25.0000 , 60.0000, 100.0000, 150.0000, 200.0000, 250.0000, 300.0000])
P = 250*np.ones(np.size(T))
write_database(T = T, P = P, nCa_cpx = 0.5, solid_solution = 'Yes', dataset = 'EQ36')
Success, your new EQ3/6 database is ready for download
<pygcc.pygcc_utils.write_database at 0x7f05e0b798d0>
A typical example of the command to generate ToughReact using 0°C to 300°C temperature range, 250 bar, and turning on the inclusion of clay minerals thermodynamics only, also specifying the location of the source database is as follows:
write_database(T = [0, 300], P = 250, clay_thermo = 'Yes', sourcedb = './database/thermo.com.tdat',
dataset = 'ToughReact', sourceformat = 'GWB')
Success, your new ToughReact database is ready for download
<pygcc.pygcc_utils.write_database at 0x7f05e0b79d50>
A typical example of the command to generate Pflotran using 0°C to 300°C temperature range, 250 bar, and turning on the inclusion of clay minerals thermodynamics only, here we can specify EQ36 sourceformat is as follows:
write_database(T = [0, 300], P = 250, clay_thermo = 'Yes', dataset = 'Pflotran',
sourceformat = 'EQ36')
Success, your new Pflotran database is ready for download
<pygcc.pygcc_utils.write_database at 0x7f05df7b6ed0>
Citation and contact information
If you use pyGeochemCalc (pygcc) for publications, consider citing the following paper:
Awolayo, A. N. and Tutolo, B. M. (2022). “PyGeochemCalc: A python package for geochemical thermodynamic calculations and software-specific thermodynamic database production from ambient to deep Earth conditions”. Chemical Geology, 606 (120984). doi:10.1016/j.chemgeo.2022.120984
If you found a bug or have questions that are not answered in this documentation, please contact:
“Adedapo Awolayo adedapo.awolayo@ucalgary.ca”
“Benjamin Tutolo benjamin.tutolo@ucalgary.ca”
References:
Wagner W, Pruß A. (2002). The IAPWS formulation 1995 for the thermodynamic properties of ordinary water substance for general and scientific use. Journal of Physical and Chemical Reference Data 31(2): 387–535. doi: 10.1063/1.1461829
Johnson J. W., Oelkers E. H., and Helgeson H. C. (1992). SUPCRT92: A software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1 to 5000 bar and 0 to 1000°C. Computers and Geosciences 18(7): 899–947. doi: 10.1016/0098-3004(92)90029-Q
Wolery, T.J., (2020). H2OI95: A Stand-Alone Fortran Code for Evaluating the IAPWS-95 Equation-of-State Model for Water (Rev. 1) (No. LLNL-TR-805304). Lawrence Livermore National Lab.(LLNL), Livermore, CA (United States).
Helgeson H.C. and Kirkham D.H., (1974), American Journal of Science, Vol.274, pp:1199-1261
International Association for the Properties of Water and Steam, IAPWS R8-97 (1997), Release on the Static Dielectric Constant of Ordinary Water Substance for Temperatures from 238 K to 873 K and Pressures up to 1000 MPa (1997), 9 pp
Fernandez D. P., Goodwin A. R. H., Lemmon E. W., Levelt Sengers J. M. H., and Williams R. C. (1997) A Formulation for the Permittivity of Water and Steam at Temperatures from 238 K to 873 K at Pressures up to 1200 MPa, including Derivatives and Debye-Hückel Coefficients. J. Phys. Chem. Ref. Data 26, 1125-1166
Robie R. A. and Hemingway B. S. (1995). Thermodynamic Properties of Minerals and Related Substances at 298.15 K and 1 Bar (105 Pascals) Pressure and at Higher Temperatures. Washington, D. C.: U. S. Geological Survey. (Bulletin 2131). doi: 10.3133/b2131
Stefánsson, A. (2001). Dissolution of primary minerals of basalt in natural waters: I. Calculation of mineral solubilities from 0 C to 350 C. Chemical Geology, 172(3-4), 225-250.
Blanc, P., Vieillard, P., Gailhanou, H., Gaboreau, S., Gaucher, É., Fialips, C. I., Madé, B. and Giffaut, E. (2015). A generalized model for predicting the thermodynamic properties of clay minerals. American journal of science, 315(8), 734-780.
Blanc, P., Gherardi, F., Vieillard, P., Marty, N. C. M., Gailhanou, H., Gaboreau, S., Letat, B., Geloni, C., Gaucher, E.C. and Madé, B. (2021). Thermodynamics for clay minerals: calculation tools and application to the case of illite/smectite interstratified minerals. Applied Geochemistry, 104986.
Kong, X. Z., Tutolo, B. M., Saar, M. O. (2013) DBCreate: A SUPCRT92-Based Program for Producing EQ3/6, TOUGHREACT, and GWB Thermodynamic Databases at User-Defined T and P. Comput. Geosci., 51, 415–417.
Johnson, J. W., and Norton, D. (1991) Critical phenomena in hydrothermal systems: state, thermodynamic, electrostatic, and transport properties of H20 in the critical region: Am. Jour. Science, v. 291, no. 6, p. 541-648
Haar, L., Gallagher, J. S., and Kell, G. S. (1984). NBS/NRC steam tables thermodynamic and transport properties and computer programs for vapor and liquid states of water in SI units. Hemisphere, Washington, D.C., 320 p
Sverjensky, D.A., Harrison, B., Azzolini, D., 2014. Water in the deep Earth: The dielectric constant and the solubilities of quartz and corundum to 60kb and 1200°C. Geochim. Cosmochim. Acta 129, 125–145. https://doi.org/10.1016/j.gca.2013.12.019
Zhang, C. and Duan, Z., 2009. “A model for C-O-H fluid in the Earth’s mantle”, Geochimica et Cosmochimica Acta, vol. 73, no. 7, pp. 2089–2102, doi:10.1016/j.gca.2009.01.021.
Zhang, Z., Duan, Z., 2005. Prediction of the PVT properties of water over wide range of temperatures and pressures from molecular dynamics simulation. Phys. Earth Planet. Inter. 149, 335–354. https://doi.org/10.1016/j.pepi.2004.11.003.
Johnson, J.W., Norton, D., 1991. Critical phenomena in hydrothermal systems: state, thermodynamic, electrostatic, and transport properties of H2O in the critical region. Am. J. Sci. 291, 541–648. https://doi.org/10.2475/ajs.291.6.541
Maier, C.G., Kelley, K.K., 1932. An equation for the representation of high-temperature heat content data. J. Am. Chem. Soc. https://doi.org/10.1021/ja01347a029
Haas, J.L., Fisher, J.R., 1976. Simultaneous evaluation and correlation of thermodynamic data. Am. J. Sci. https://doi.org/10.2475/ajs.276.4.525
Helgeson, H.C., Delany, J.M., Nesbitt, H.W., Bird, D.K., 1978. Summary and critique of the thermodynamic properties of the rock-forming minerals. Am. J. Sci. 278A, 229
Holland, T. and Powell, R., 2011. An improved and extended internally consistent thermodynamic dataset for phases of petrological interest, involving a new equation of state for solids. Journal of Metamorphic Geology. 29, 333–383.
Holland, T.J.B. and Powell, R., 1998. An internally-consistent thermodynamic dataset for phases of petrological interest. Journal of Metamorphic Geology, 16, 309–344.
Zimmer, K., Zhang, Y.L., Lu, P., Chen, Y.Y., Zhang, G.R., Dalkilic, M. and Zhu, C. (2016) SUPCRTBL: A revised and extended thermodynamic dataset and software package of SUPCRT92. Computer and Geosciences 90:97-111
Thank you and have fun utilizing pyGeochemCalc